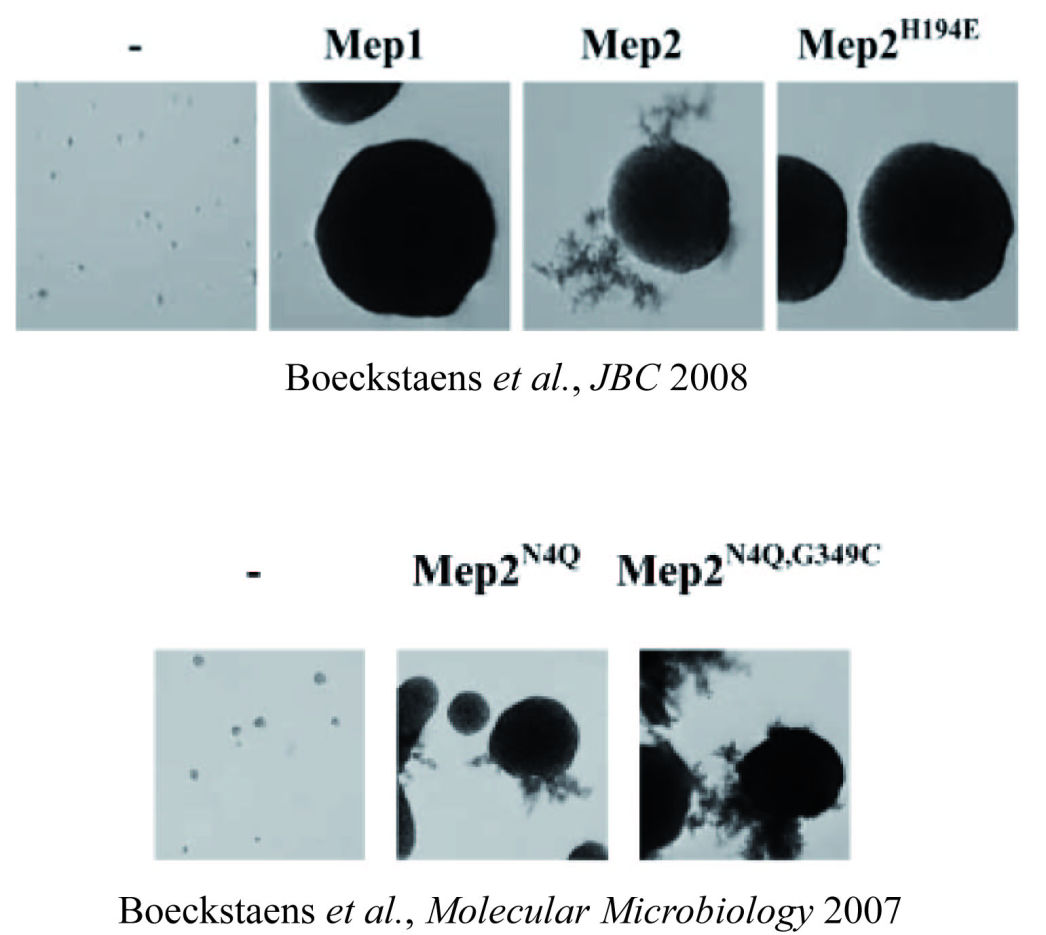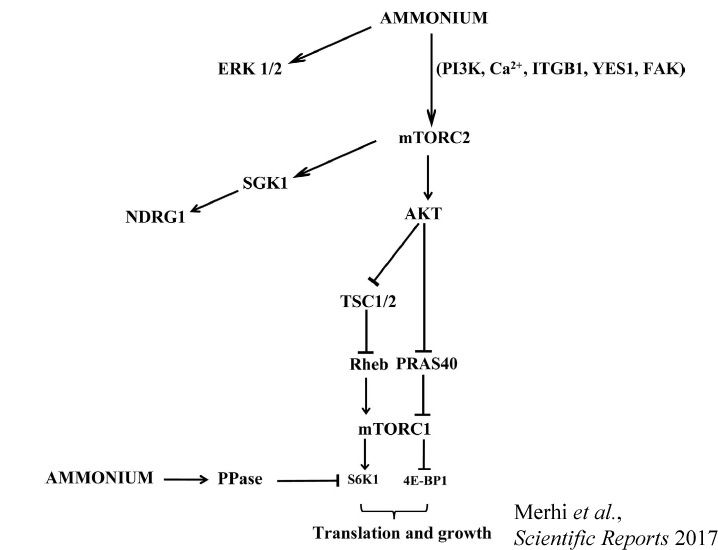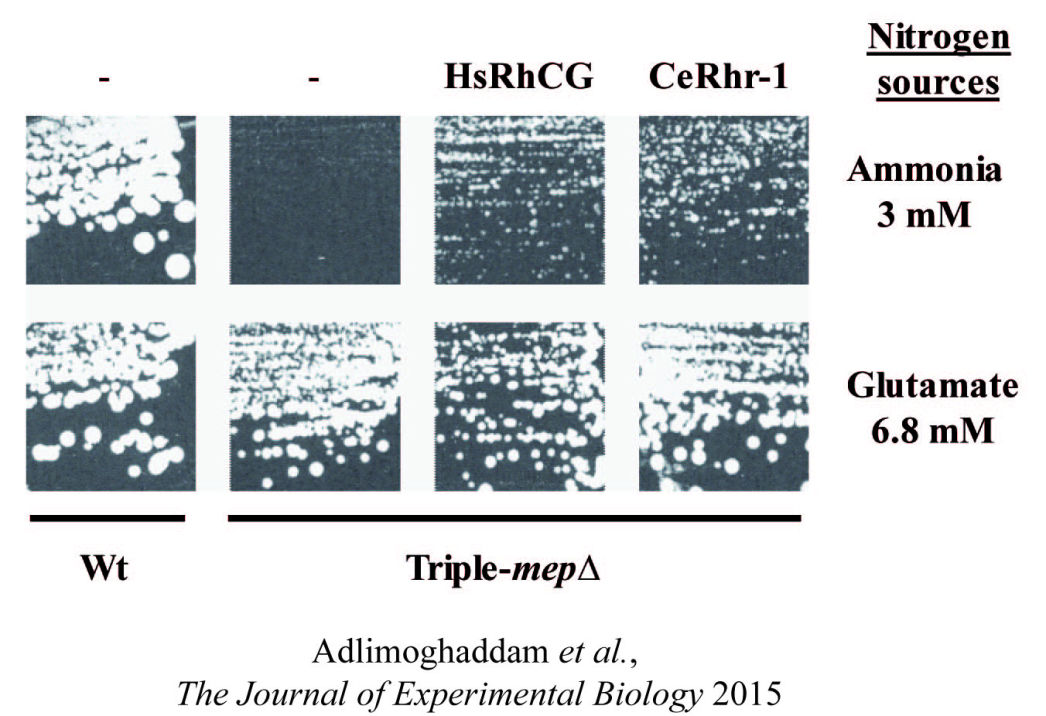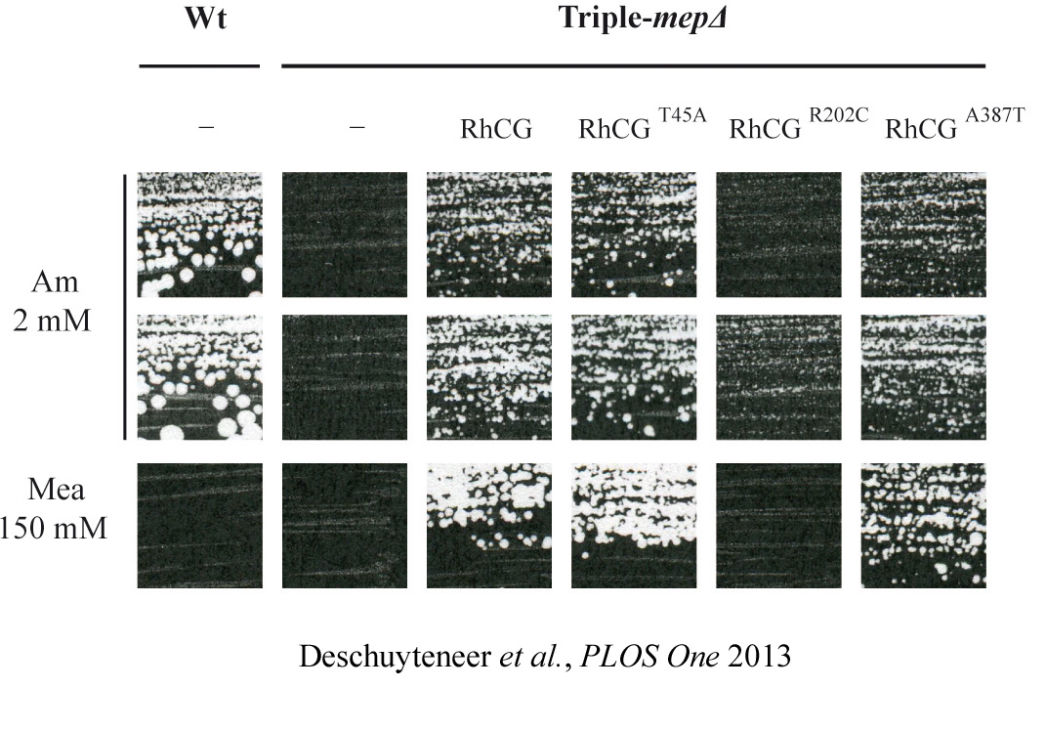
Deciphering the mechanisms and the signaling pathways involved in the activity regulation of eukaryotic Mep-Amt-Rh members remained an open field. We showed for the first time that, in yeast, the conserved TORC1 complex, controlling cell growth, is able to directly control ammonium permeability in a fast and flexible response to environmental perturbation. We highlight a mechanism involving a phosphorylation/dephosphorylation balance of a serine residue (S457) in an auto-inhibitory domain of the C-terminus of Mep2, fine-tuning its transport activity. The TORC1 effector kinase Npr1 and the two phosphatases Psr1 and Psr2 control this phosphorylation balance.
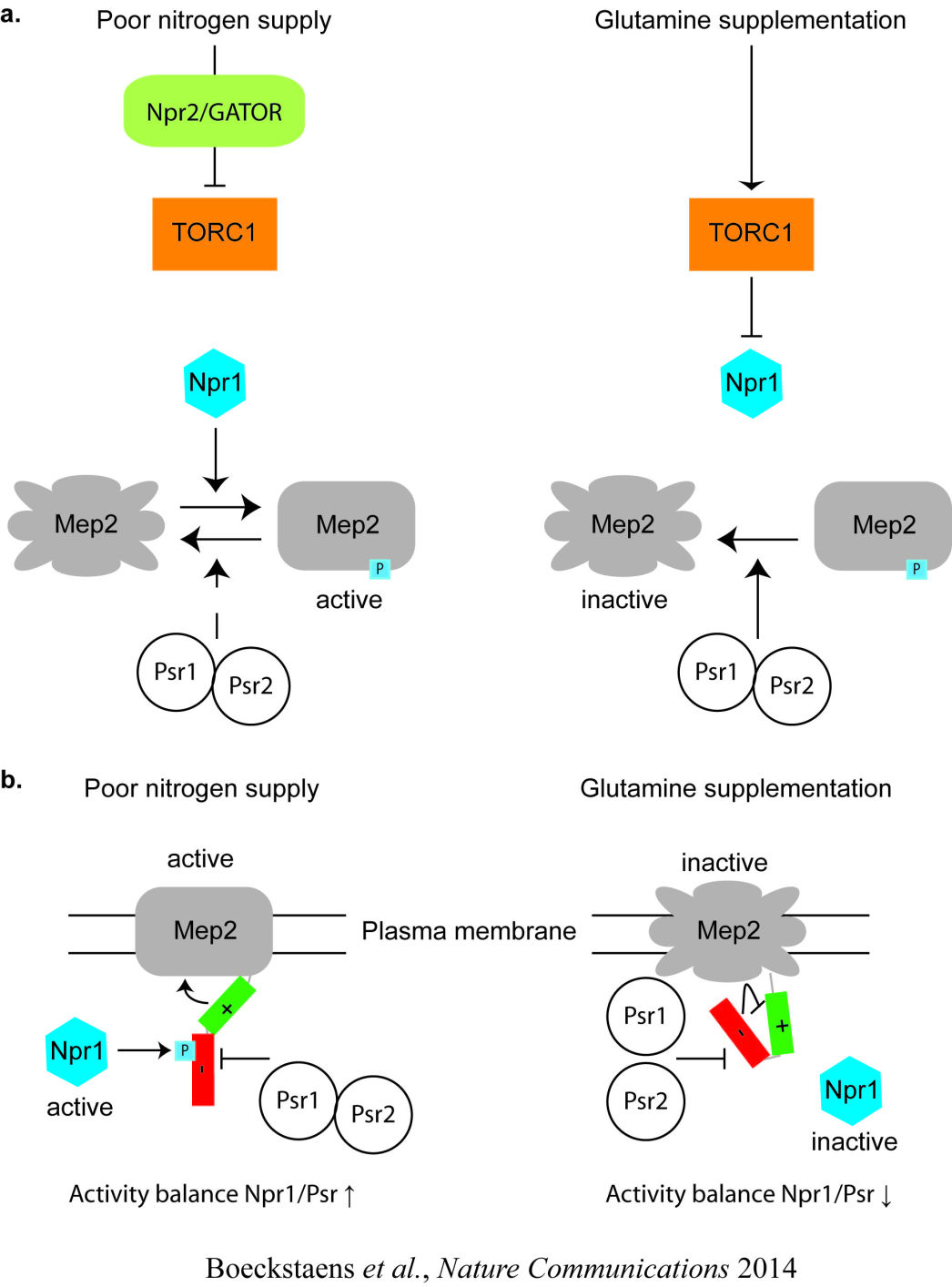
We further identified a conserved signaling protein Npr2, a tumor suppressor in humans, transmitting the nitrogen signal to TORC1. Moreover, in a work
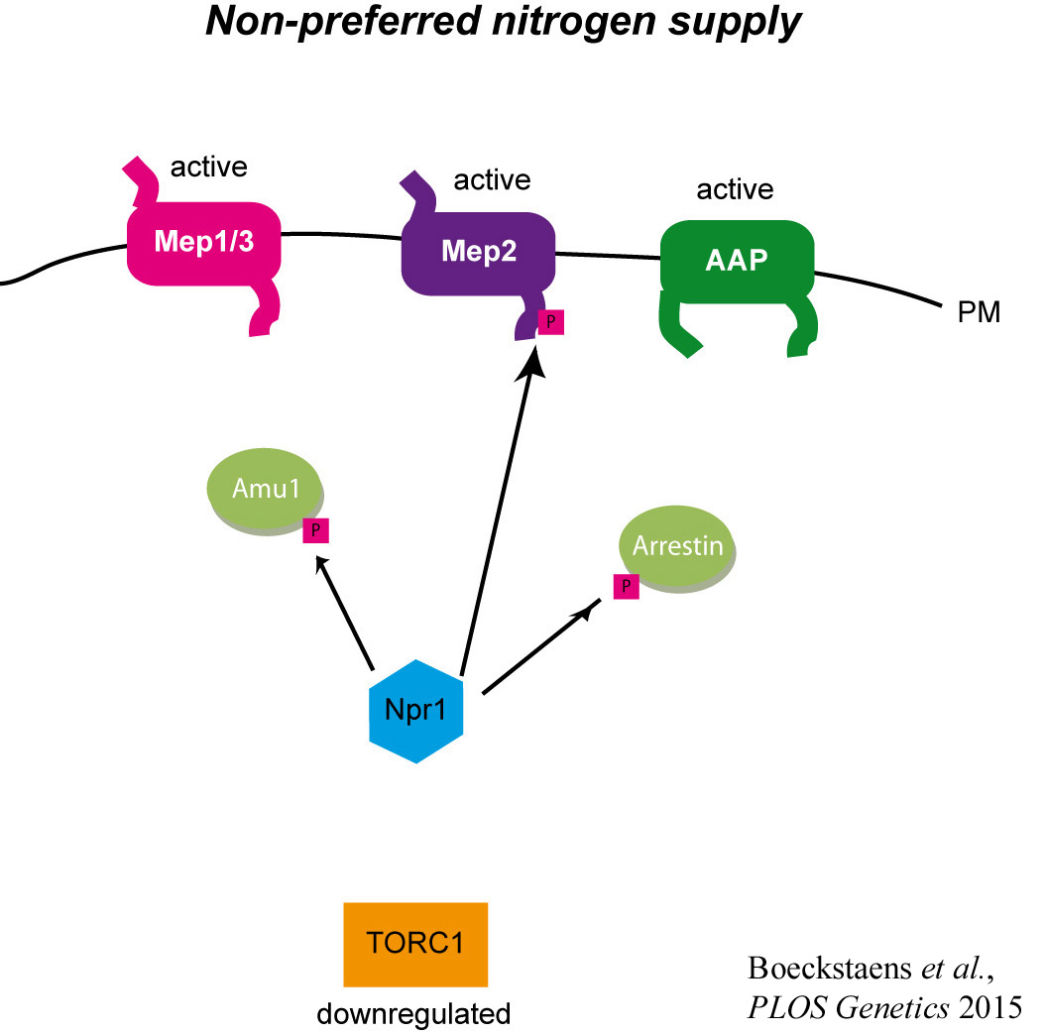
published in 2015 in PLOS Genetics, we showed that TORC1-Npr1 regulates the inherent activity of the remaining yeast Mep1 and Mep3 ammonium transport proteins by a different process. It involves the phospho-silencing of an intermediate negative regulator Amu1/Par32, a functional orphan, able to directly inhibit Mep1 and Mep3.